In 2017, the European Atherosclerosis Society (EAS) published a paper called "Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel" (1).
Although there are many fallacies in this paper, one its most misleading aspects is the constant use of ecological associations. The authors not only apply these associations to cardiovascular events but also to a measure of atherosclerosis.
For this post, I will use the EAS analysis and show how strong ecological correlations between LDL and atherosclerosis can be completely explained by simple biases.
Images Can Be Deceiving
On page 2465 of the EAS paper, the authors state (1):
Intravascular ultrasound [IVUS] studies of coronary atherosclerosis involving statin-treated patients have consistently demonstrated that progression of coronary atherosclerotic plaque volume can be substantially arrested at achieved LDL-C levels of ∼1.8 mmol/L (70 mg/dL).
To support this statement, the following image was provided:
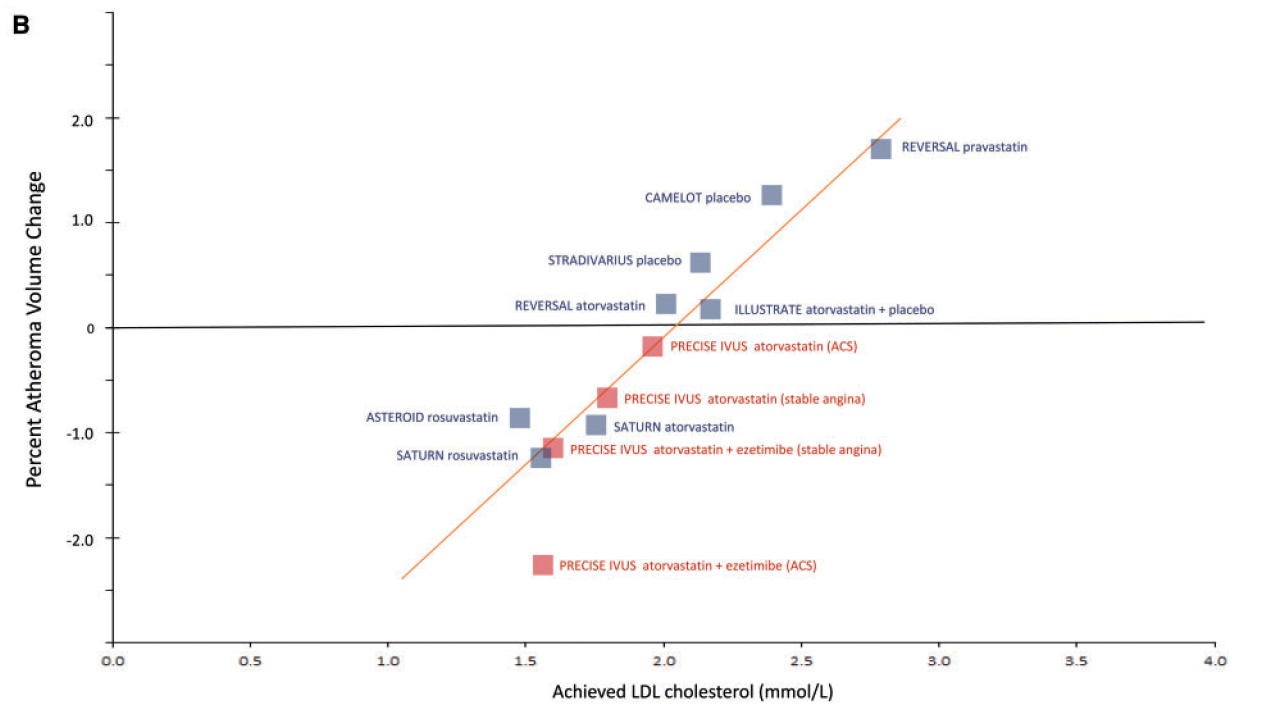 (Image: An ecological association between LDL and IVUS-determined atherosclerosis. Note that the analysis is based on aggregated or group-level data and thus qualifies as "ecological.")
(Image: An ecological association between LDL and IVUS-determined atherosclerosis. Note that the analysis is based on aggregated or group-level data and thus qualifies as "ecological.")
At face value, this image looks impressive. It appears to show a strong correlation between LDL levels and progression of atherosclerosis. Yet, no matter how impressive the correlation looks, we still cannot conclude that lower LDL causes a decrease in atherosclerosis, for correlation does not imply causation.
In fact, as convincing as this image may seem on the surface, I will assert that it is spurious. There is actually no strong correlation (and no causation) between LDL and atherosclerosis. What we see in the image is likely due to biases that come with ecological group-level data (i.e., confounding and aggregation bias).
As an epidemiological text explains (2):
In general, ecological studies are attractive because they are easy to do, especially if the routine data are readily available, but they can be difficult to interpret. The populations [or groups] being compared may well differ in ways other than their exposure to the factor of interest and it is possible that something else that is related to the exposure is actually responsible . . . Another problem with this type of study is that an observed association between variables at the group level might not represent the association at the individual level.
Indeed, it is long known that relationships between groups do not necessarily hold for individuals (3,4).
To get an idea of this ecological bias, let us look at some examples outside the EAS paper.
Examples of Ecological Bias
Rosen and colleagues nicely illustrated the perils of ecological associations using a simple hypothetical example. To quote (5):
Let us imagine three groups: A, B and C (Figure 1). These may denote counties, municipalities, occupations, etc.
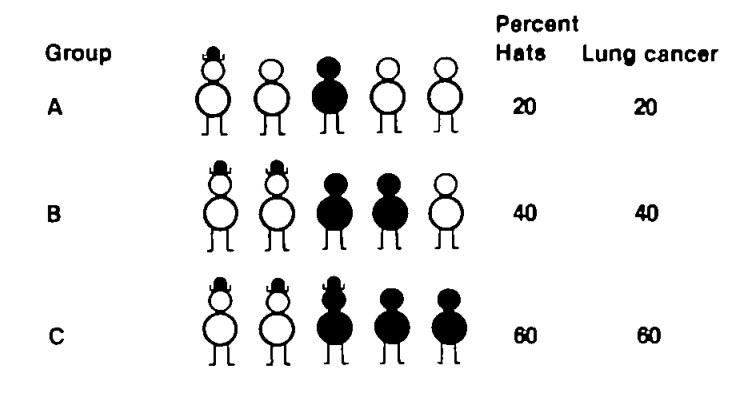 (Image: Figure 1 taken from reference 5)
(Image: Figure 1 taken from reference 5)
Group A consists of five individuals, one of whom wears a hat and one has lung cancer (black figure). This means that one out of five—ie 20%— wear hats and 20% have lung cancer in group A. Group B and C are then computed accordingly. Now, if we make a simple correlation analysis, we get a 100% correlation on the group level.
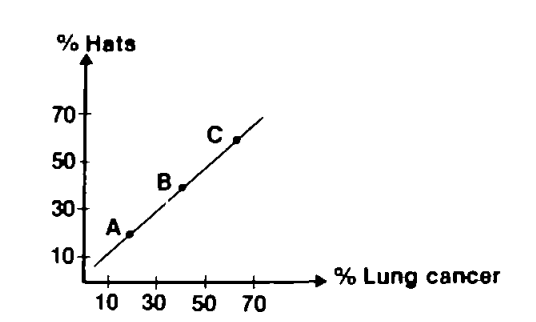
If, however, each individual is studied separately, those who wear hats and those who develop lung cancer are generally not one and the same.
In other words, there is a perfect group-level correlation between hat wearing and lung cancer, but this correlation does not apply at the individual level, because most individuals who wear hats do not have lung cancer. Thus, the group-level correlation gives the wrong impression.
To give a real-world example, Bots et al. showed a strong correlation between LDL and CRP (an inflammatory marker) when group-level information was used (6):
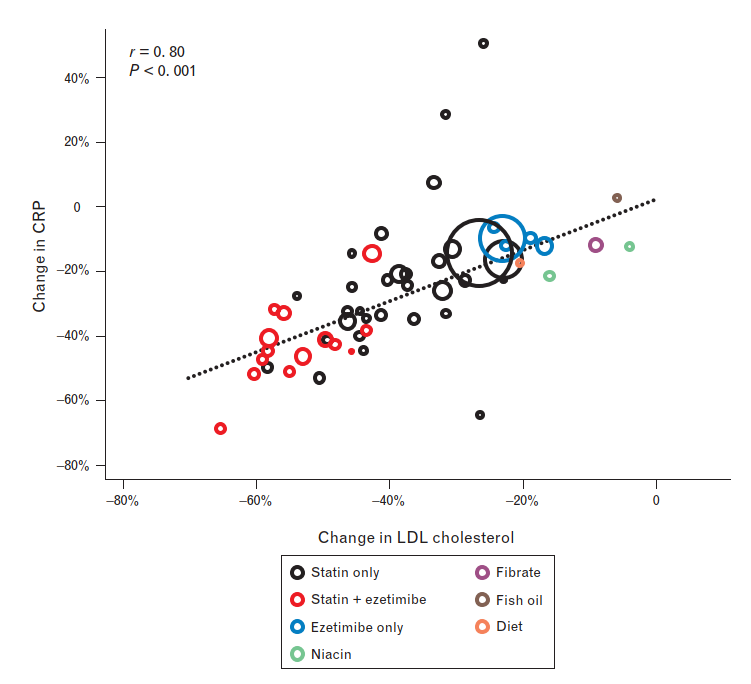 (Image: This group-level correlation makes LDL look like an inflammation-inducing substance! Taken from reference 6)
(Image: This group-level correlation makes LDL look like an inflammation-inducing substance! Taken from reference 6)
But, when they looked at a study using individual-level data, no correlation was found at all:
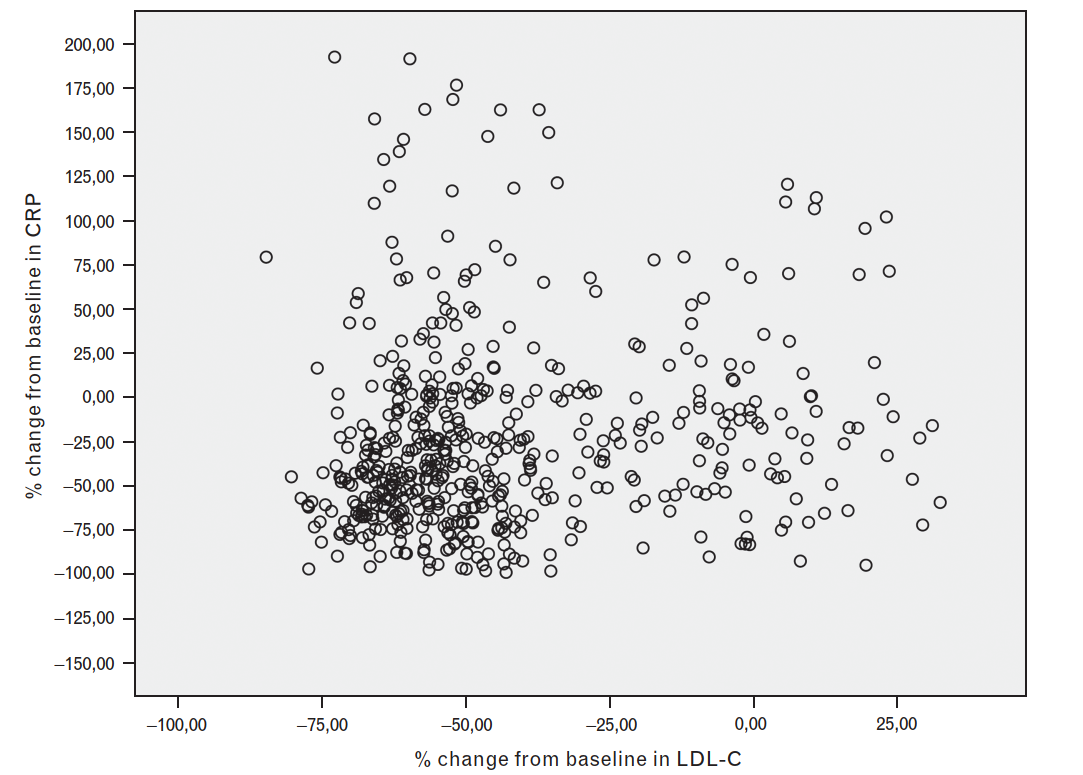 (Image: No correlation between LDL and CRP on an individual level, the correct conclusion)
(Image: No correlation between LDL and CRP on an individual level, the correct conclusion)
Group-level data can exaggerate relationships, produce a relationship when there is really none, or even produce a relationship in the opposite direction of the truth. The latter is shown using blood pressure and creatinine in a 2021 paper aptly titled "Be careful with ecological associations" (7):
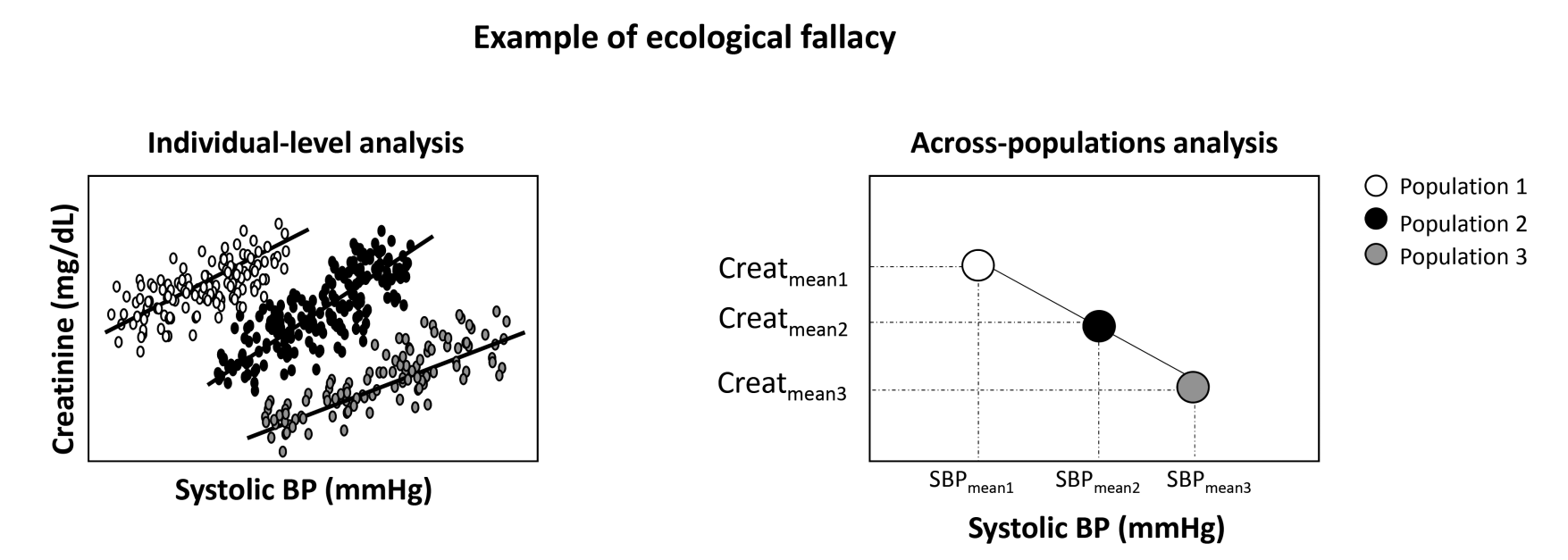 (Image: Ecological associations can be so misleading that relations may completely reverse)
(Image: Ecological associations can be so misleading that relations may completely reverse)
Therefore, to begin arguing for causation, we need to look at individual-level data where confounding bias is less and aggregation bias (the ecological fallacy) is avoided (8):
Researchers must first look at individual correlations to link outcomes (to avoid the ecological fallacy), and then work further to clearly establish causality.
Now back to the EAS paper . . .
Digging Their Own Hole
The studies included in the EAS analysis itself provide good examples of why ecological associations cannot be trusted.
Of the seven trials included in their analysis, six had published analyses at an individual level or at least provided within-study correlations. These studies are ASTEROID, REVERSAL, SATURN, CAMELOT, ILLUSTRATE, and PRECISE IVUS. Contrary to the EAS ecological analysis, these six studies found weak to non-existent relations between LDL and atherosclerosis progression.
ASTEROID
Within the ASTEROID study itself, there was actually no correlation between atherosclerosis regression and LDL (9). The authors explicitly stated:
The on-treatment atheroma volume, change in atheroma volume, and high percentage of subjects with atheroma regression did not differ by the achieved LDL cholesterol . . . Atheroma regression occurred in most patients and was not linked to the LDL cholesterol achieved.
They further acknowledged that statins have other effects aside from LDL lowering that may explain the results (9):
These multiple mechanisms of the beneficial effects of statins could explain why event reduction and lesion modification correlated poorly with LDL cholesterol lowering alone.
REVERSAL
The REVERSAL study did find a correlation, but the authors admitted that the relation was weak (note: they may have found no correlation if examined within each statin group separately) (10):
Since the levels of both CRP and LDL cholesterol showed relatively weak correlations . . . this analysis demonstrates that biomarkers can account for only a small fraction of the observed progression rate.
Moreover, like ASTEROID, there was no association between LDL and atherosclerosis regression:
Atherosclerosis regressed in patients with the greatest reduction in CRP levels, but not in those with the greatest reduction in LDL cholesterol levels.
SATURN
The title of one paper from SATURN is self-explanatory (11):
C-Reactive Protein, but not Low-Density Lipoprotein Cholesterol Levels, Associate With Coronary Atheroma Regression and Cardiovascular Events
I also found another paper from SATURN with a table showing no relation for LDL and any of the atherosclerotic measures (12):
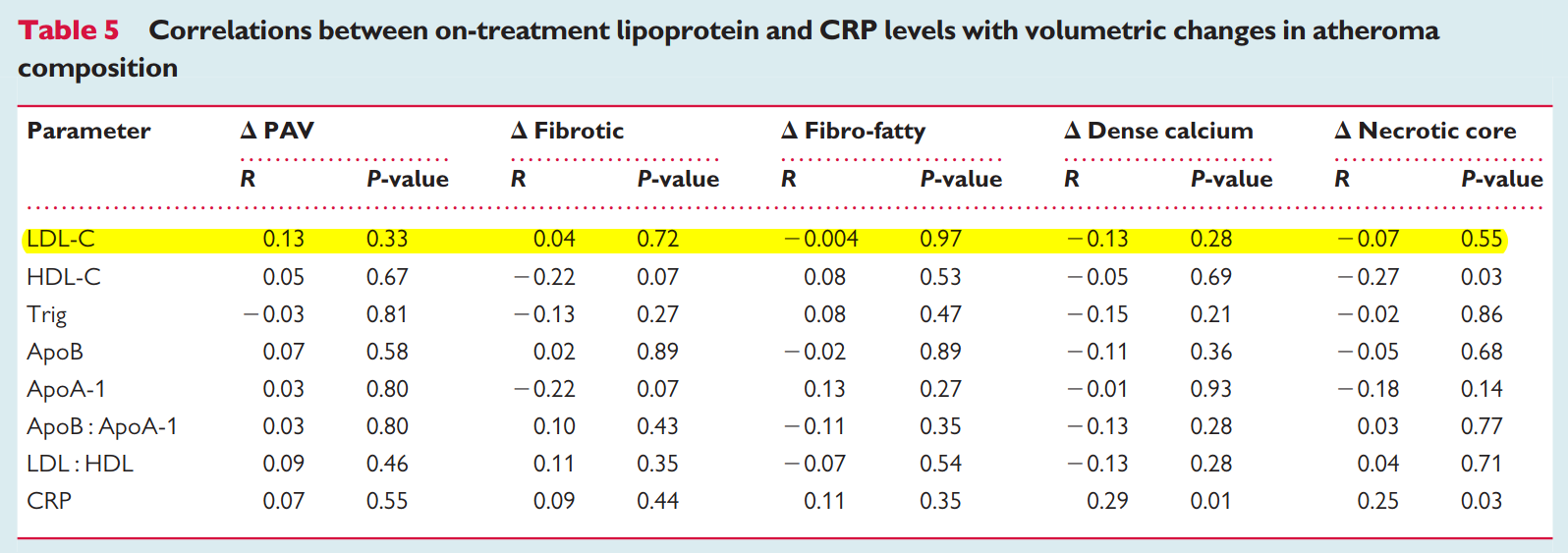
ILLUTRATE
The ILLUTRATE trial found no association between LDL and atherosclerosis (13):
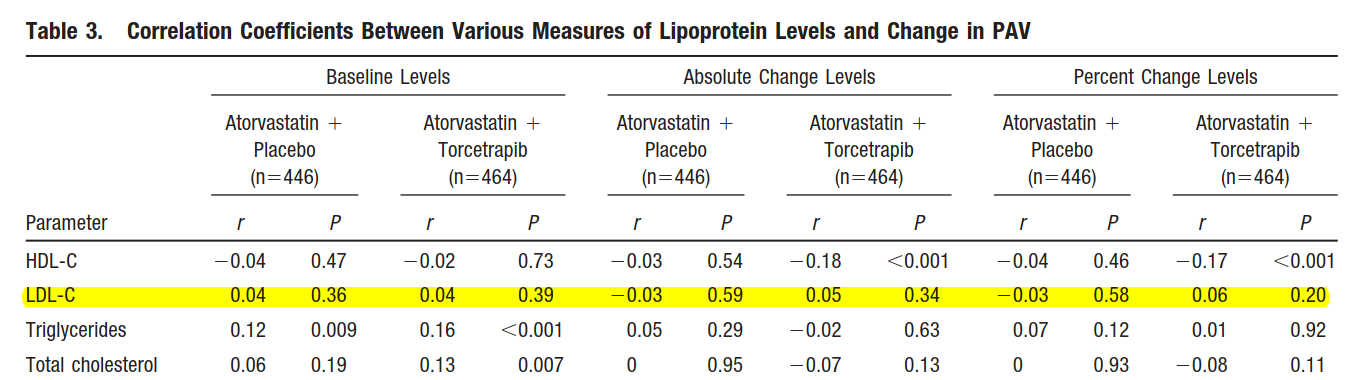
On multivariable analysis, the change in LDL still did not correlate with atherosclerosis, but the change in HDL was.
CAMELOT
The CAMELOT study demonstrated no association (14):
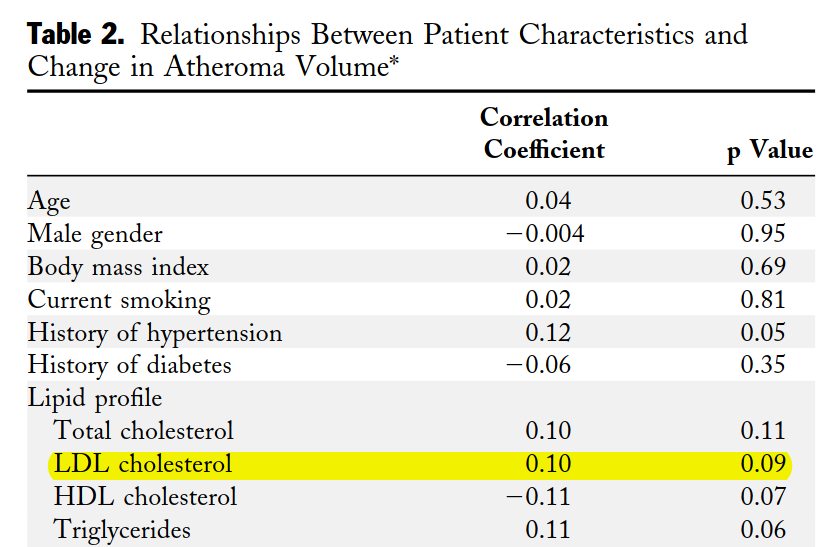
PRECISE-IVUS
For the most part, the PRECISE-IVUS study showed no association between LDL and atherosclerosis. This is nicely illustrated in the following scatterplot of the whole cohort (15):
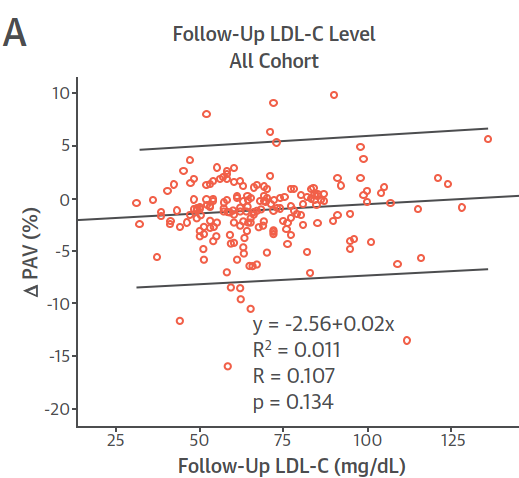 (Image: In contradiction to the EAS analysis, there is really no relation between LDL and atherosclerosis)
(Image: In contradiction to the EAS analysis, there is really no relation between LDL and atherosclerosis)
Crea and Niccoli also noted the lack of relation in PRECISE-IVUS, stating (16):
At first glance, the lower mean LDL-C level achieved with combination therapy than with monotherapy (63.2 mg/dl vs. 73.3 mg/dl) seems to account for the enhanced regression associated with the former. However, linear regression analysis failed to show an association between achieved LDL-C levels and PAV changes . . . Thus, cholesterol lowering itself does not seem to explain the greater reduction of PAV . . .
Tip of the Ice Berg
The studies included in the EAS analyses are not the only IVUS studies showing no relation between LDL and atherosclerosis. Eleven years before the EAS publication, Nicholls et al. demonstrated the same disconnect (17):
Low-density lipoprotein and C-reactive protein were not significant predictors of greater disease burden.
It is also easy to find others published the very same year as the EAS analysis. For example, Kwon et al. (2017) found no association (18), and the scatterplot from Udea et al. (2017) clearly shows none (19):
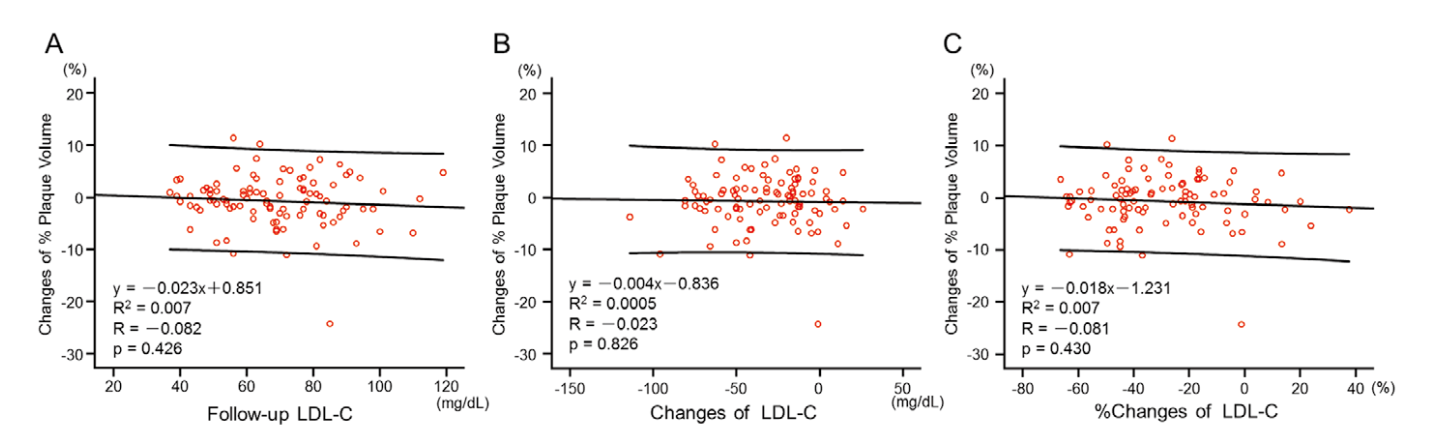 (Image: No association between LDL and atherosclerosis on an individual level)
(Image: No association between LDL and atherosclerosis on an individual level)
Even some group-level analyses do not find any association, but these are rarely cited (20):
Statin-induced reduction in PV [plaque volume] was not found to be significantly associated with LDL-C reductions.
I also cite many more studies in "The Lipid Hypothesis of Atherosclerosis: Why LDL Particles and Cholesterol Do Not Cause Disease" (found here or here).
Conclusions
It is easy to find numerous silly correlations between variables, such as the strong correlation between IKEA furniture stores and Nobel laureates (21):
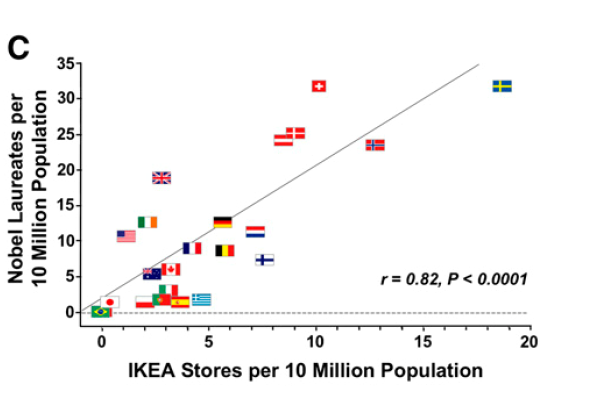 The true danger, however, are those correlations that have a bit of superficial plausibility about them. In the case of LDL, group-level correlations are given credibility despite being subjected to the same severe biases as all those silly examples we may find. And to make things worse, major contradictions are simply ignored.
The true danger, however, are those correlations that have a bit of superficial plausibility about them. In the case of LDL, group-level correlations are given credibility despite being subjected to the same severe biases as all those silly examples we may find. And to make things worse, major contradictions are simply ignored.
Thus, the simple fact is: There is little to no relation between LDL and atherosclerosis on an individual level. A misleading ecological analysis conducted by a "consensus panel" with major conflicts of interest (1,22) doesn't change that.
References
1) Ference, B. A., Ginsberg, H. N., Graham, I., Ray, K. K., Packard, C. J., Bruckert, E., ... & Catapano, A. L. (2017). Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European heart journal, 38(32), 2459-2472.
2) Webb, P., Bain, C., & Page, A. (2017). Essential epidemiology: an introduction for students and health professionals. Cambridge University Press.
3) Freedman, D. A. (1999). Ecological inference and the ecological fallacy. International Encyclopedia of the social & Behavioral sciences, 6(4027-4030), 1-7.
4) Higgins, J. P., López-López, J. A., & Aloe, A. M. (2020). Meta-regression. In Handbook of meta-analysis (pp. 129-150). Chapman and Hall/CRC.
5) ROSÉN, M., NYSTRÖM, L., & WALL, S. (1985). Guidelines for regional mortality analysis: an epidemiological approach to health planning. International journal of epidemiology, 14(2), 293-299.
6) Bots, M. L., Taylor, A. J., Kastelein, J. J., Peters, S. A., Den Ruijter, H. M., Tegeler, C. H., ... & Grobbee, D. E. (2012). Rate of change in carotid intima–media thickness and vascular events: meta-analyses can not solve all the issues. A point of view.
7) Roumeliotis, S., Abd ElHafeez, S., Jager, K. J., Dekker, F. W., Stel, V. S., Pitino, A., ... & Tripepi, G. (2021). Be careful with ecological associations. Nephrology, 26(6), 501-505.
8) Cragg, J. J., Kramer, J. L., Borisoff, J. F., Patrick, D. M., & Ramer, M. S. (2019). Ecological fallacy as a novel risk factor for poor translation in neuroscience research: A systematic review and simulation study. European journal of clinical investigation, 49(2), e13045.
9) Wiviott, S. D., Mohanavelu, S., Raichlen, J. S., Cain, V. A., Nissen, S. E., & Libby, P. (2009). Safety and efficacy of achieving very low low-density lipoprotein cholesterol levels with rosuvastatin 40 mg daily (from the ASTEROID Study). The American journal of cardiology, 104(1), 29-35.
10) Puri, R., Nissen, S. E., Libby, P., Shao, M., Ballantyne, C. M., Barter, P. J., ... & Nicholls, S. J. (2013). C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation, 128(22), 2395-2403.
11) Nissen, S. E., Tuzcu, E. M., Schoenhagen, P., Crowe, T., Sasiela, W. J., Tsai, J., ... & Ganz, P. (2005). Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. New England Journal of Medicine, 352(1), 29-38.
12) Puri, R., Libby, P., Nissen, S. E., Wolski, K., Ballantyne, C. M., Barter, P. J., ... & Nicholls, S. J. (2014). Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. European Heart Journal–Cardiovascular Imaging, 15(4), 380-388.
13) Nicholls, S. J., Tuzcu, E. M., Brennan, D. M., Tardif, J. C., & Nissen, S. E. (2008). Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation, 118(24), 2506-2514.
14) Sipahi, I., Tuzcu, E. M., Schoenhagen, P., Wolski, K. E., Nicholls, S. J., Balog, C., ... & Nissen, S. E. (2006). Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. Journal of the American College of Cardiology, 48(4), 833-838.
15) Tsujita, K., Sugiyama, S., Sumida, H., Shimomura, H., Yamashita, T., Yamanaga, K., ... & PRECISE–IVUS Investigators. (2015). Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. Journal of the American College of Cardiology, 66(5), 495-507.
16) Crea, F., & Niccoli, G. (2015). Ezetimibe and plaque regression: cholesterol lowering or pleiotropic effects?.
17) Nicholls, S. J., Tuzcu, E. M., Crowe, T., Sipahi, I., Schoenhagen, P., Kapadia, S., ... & Nissen, S. E. (2006). Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. Journal of the American College of Cardiology, 47(10), 1967-1975.
18) Kwon, O., Kang, S. J., Kang, S. H., Lee, P. H., Yun, S. C., Ahn, J. M., ... & Park, S. J. (2017). Relationship between serum inflammatory marker levels and the dynamic changes in coronary plaque characteristics after statin therapy. Circulation: Cardiovascular Imaging, 10(7), e005934.
19) Ueda, Y., Hiro, T., Hirayama, A., Komatsu, S., Matsuoka, H., Takayama, T., ... & ZIPANGU Investigators. (2017). Effect of Ezetimibe on Stabilization and Regression of Intracoronary Plaque―The ZIPANGU Study
20) Banach, M., Serban, C., Sahebkar, A., Mikhailidis, D. P., Ursoniu, S., Ray, K. K., ... & Serruys, P. W. (2015). Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC medicine, 13(1), 1-21.
21) Maurage, P., Heeren, A., & Pesenti, M. (2013). Does chocolate consumption really boost Nobel award chances? The peril of over-interpreting correlations in health studies. The Journal of Nutrition, 143(6), 931-933.
22) van Bruggen, F. H., Nijhuis, G. B. J., Zuidema, S. U., & Luijendijk, H. J. (2021). Response to letter to the editor re:‘serious adverse events and deaths in PCSK9 inhibitor trials reported on ClinicalTrials. gov: a systematic review’. Expert Review of Clinical Pharmacology, 14(2), 283-284.
