In 2016, Silverman and colleagues published a meta-analysis and meta-regression evaluating the association between LDL cholesterol and cardiovascular outcomes (1).
Based on meta-regressions, they concluded:
The use of statin and nonstatin therapies that act via upregulation of LDL receptor expression to reduce LDL-C were associated with similar RRs [relative risks] of major vascular events per change in LDL-C. Lower achieved LDL-C levels were associated with lower rates of major coronary events.
But as noted before, meta-analyses are shot through with biases, rendering such evidence very often unreliable (2).
Meta-analyses are, for the most part, retrospective exercises conducted by researchers (often with conflicts of interest) who already know the results of studies in the field (3).
As such, those conducting meta-analyses can make decisions (inclusion/exclusion criteria, statistical methods, endpoints, etc.) to get any result they want.
As Shrier et al. stated (4):
The evidence-based movement has proposed that a systematic review with a meta-analysis of RCTs [randomized clinical trials] on a topic provides the strongest evidence of support and that widespread adoption of its results should lead to improved patient care. However, our results suggest that the interpretation of a meta-analysis (and therefore recommendations) are subjective and therefore depend on who conducts or interprets the meta-analysis.
These concerns also extend to the meta-regression — a statistical method used to analyze potential associations between study-level characteristics and effect estimates.
Silverman et al., for instance, used meta-regressions to explore whether LDL reductions (at the study level) might explain variations in cardiovascular risk (at the study level).
But just as most meta-analyses are riddled with biases, so too are meta-regressions. And the Silverman review is no exception.
To illustrate, let us recap the limitations of meta-regressions.
Limitation #1: Meta-Regressions Are Ecological Analyses
Meta-regressions can be deceptive because they often involve randomized trials — a study design that is generally considered the "gold standard" for determining causes.
We could see this in the Silverman analysis, which included "randomized" trials on different drug classes:
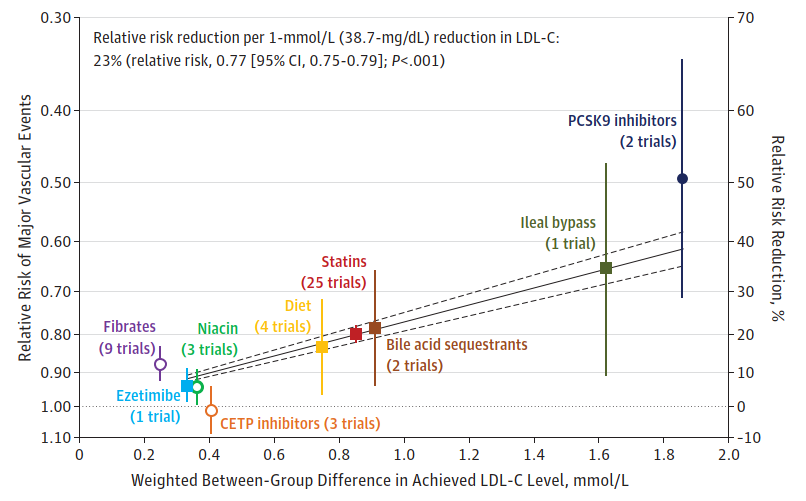
Upon seeing this graph (and others like it), the thought process of the reader may look similar to the following:
"Since the analysis includes randomized trials, it must be strong evidence for a cause-and-effect relationship between LDL and cardiovascular events."
But this would be a mistake.
The fact that the meta-regression includes randomized trials is irrelevant. The benefits of randomization are lost once the data are analyzed across trials.
To quote Spineli and Pandis (5):
The findings of subgroup analysis and meta-regression should be interpreted with caution because of their observational nature. Although patients are randomly allocated to one intervention or another within a clinical trial, they are not randomly “allocated” across the trials included in the subgroup and meta-regression analyses. Therefore, subgroup analysis and meta-regression suffer from the same problems and pitfalls as observational studies, such as confounding.
And this is not controversial. Even Cochrane's handbook explicitly states that meta-regressions are entirely observational in their nature (6).
Furthermore, meta-regressions are prone to aggregation bias (7-9). Relationships between variables at a higher level (across trials) may not hold at a lower level (within trials or individuals).
Thus, meta-regressions are an extremely weak source of epidemiologic evidence (10), should be viewed with great caution (11), and should be interpreted as hypothesis generating only (12).
Some Examples
In 2006, Hayward, Hofer, and Vijan illustrated how meta-regressions can exaggerate relationships (10).
They provided the following graph, which makes LDL appear as a good marker of cardiovascular risk:
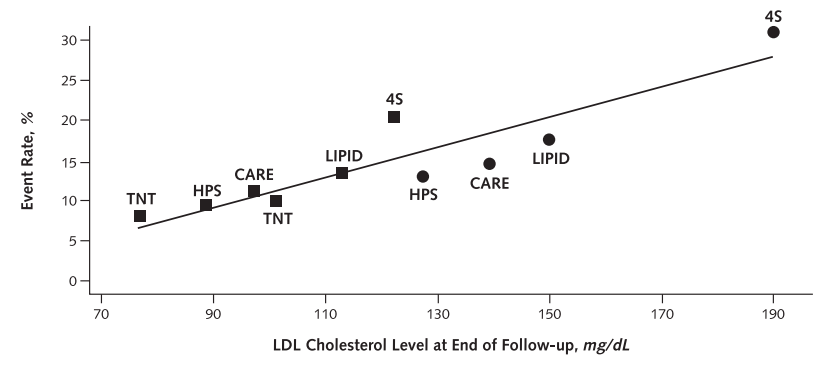
But they noted that these studies differ in many ways, such as time period, country, baseline risk, crossover, and treatment adherence rates.
Moreover, when controlling for some of these differences by using each study as its own control, the relationship between LDL and cardiovascular events changes substantially, becoming less strong and less uniform:

In the following graph from Silverman's analysis, we find a similar result. For almost all trials, when each population is used as its own control (the added black lines), the lines become shallower, sometimes much shallower than the main regression lines:
 (Image: In almost every case, the association between LDL and CHD is weaker than what the regression line predicts, suggesting systematic biases across trials. For 19 out of 24 trials, the true estimated risk reduction is 22 to 83% less than what is predicted)
(Image: In almost every case, the association between LDL and CHD is weaker than what the regression line predicts, suggesting systematic biases across trials. For 19 out of 24 trials, the true estimated risk reduction is 22 to 83% less than what is predicted)
But even these better-controlled analyses have limitations.
To be valid, we must also control for methodological issues, changes in other potential mechanisms of statin therapy, and changes in other lipids/lipoproteins (VLDL remnants, Lp(a), oxidized LDL, small dense LDL, etc.).
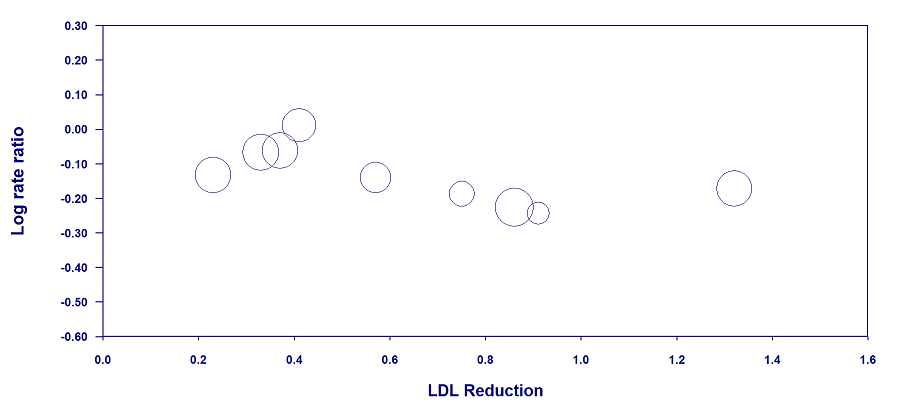
Thus, meta-regressions can exaggerate relationships due to inherent biases or create relationships that do not exist at the individual level:
 (Image: Taken from reference 9. Based on study-level data, the left graph shows a strong relationship between LDL changes and CRP changes. Yet, the right graph of almost 1000 individuals shows none)
(Image: Taken from reference 9. Based on study-level data, the left graph shows a strong relationship between LDL changes and CRP changes. Yet, the right graph of almost 1000 individuals shows none)
In their paper, Silverman et al. admit that analyses are "at the trial level" and that background therapy "may account for some of the difference in absolute event rates between trials."
But Silverman et al. also underestimate the extent of these biases and fail to explicitly mention that such analyses do not support cause-and-effect relationships, do not apply to individuals, and should be viewed with caution.
Limitation #2: Linear Assumptions
Another problem with many meta-regressions is the assumption of a linear relationship (13).
Strict linear relationships are implausible because they allow for cardiovascular event rates of 0% (or lower) and 100% (or greater) [14]. Furthermore, biology is neither linear nor simple (15).
In lipid-lowering trials, cardiovascular event rates always increase over time, regardless of the LDL level (i.e., there is no 0%). And even in high-risk populations, cardiovascular event rates do not reach anywhere near 100% (implying that strict log-linear relationships are implausible also).
Take the Silverman graph below (Figure 4 in the paper):
 (Image: Figure 4)
(Image: Figure 4)
This graph pretends to show a linear relationship: as LDL decreases, the risk decreases without any diminishing benefit (i.e., there is a constant change in the absolute risk for each unit change in the achieved LDL level).
But besides the systematic biases (as we saw in the last section), the regression lines predict negative event rates for a few trials — an impossibility.
Therefore, we should discard the linear model and replace it with a more flexible model.
In Silverman's graph, the ecological relationship for secondary prevention trials (the orange squares) might be better represented by the following curve:

(Image: Taken from reference 14. Unlike Silverman's graph, note how the curve starts to flatten around an LDL level of 100 mg/dL or 2.6 mmol/L)
Similarly, the ecological association for the primary prevention trials (the teal-colored circles in Silverman's graph) appears flat over a wide range:
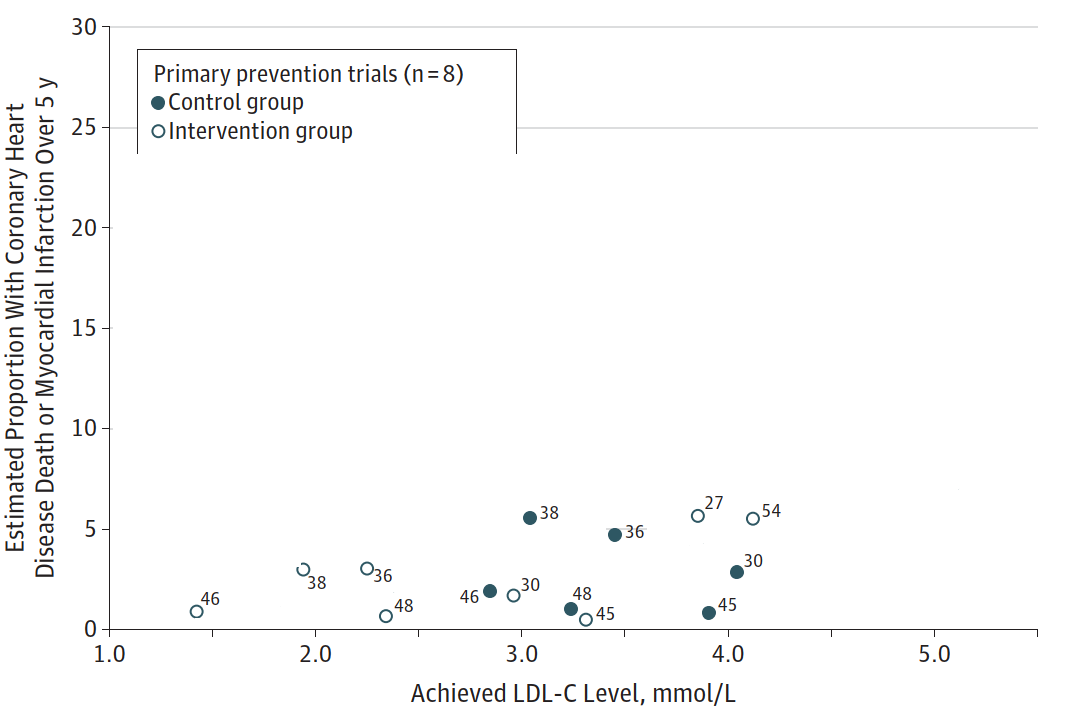
(Image: For clarity, I've erased the secondary prevention trials and the outlier primary prevention trials)
The relationship is so weak, that the control group of trial #45 has a similar event rate as the drug group of trial #46 despite vastly different LDL levels (151 mg/dL vs. 55 mg/dL).
Indeed, the epidemiological relationship between LDL and cardiovascular or mortality outcomes is often discontinuous or non-linear, and even absent at times (16):
 (Images: Taken from epidemiological cohorts in references 17 to 23)
(Images: Taken from epidemiological cohorts in references 17 to 23)
And these curves are consistent with many trials included in Silverman's meta-regression.
The Primary Prevention Trials
For the primary prevention trials in Silverman's graph (Figure 4 above), almost all had analyses evaluating lipids in relation to cardiovascular events. These include WOSCOPS, AFCAPS, MEGA, LRC-CPPT, CARDS, ALLHAT, and JUPITER.
Remarkably, virtually all analyses from these trials did not support a so-called "independent" linear relationship between LDL and cardiovascular events.
Contrary to Silverman's analysis, the WOSCOPS investigators stated that the relationship between the reduction of LDL and reduction of risk was not linear (24).
The WOSCOPS study, in fact, found no evidence of a relationship between baseline LDL or the change in LDL and CHD risk (25):
Although LDL cholesterol level is currently used to select patients for statin therapy and to monitor treatment response, it was notable that neither baseline nor change in LDL cholesterol predicted future coronary events . . . primary endpoint reduction was similar across quarters of change in LDL cholesterol.
 (Image: Taken from reference 25)
(Image: Taken from reference 25)
A similar lack of relationship was found in AFCAPS. The investigators noted little relation between on-treatment LDL and CHD risk, and that LDL is not predictive unless considered in conjunction with HDL-C (26):
Interestingly, LDL-C and TC did not achieve significance as predictors of risk either at baseline or at year 1.
For the MEGA trial, there was no linear relation between on-treatment LDL levels and cardiovascular event rates (27). Also, baseline LDL did not have a clear association with CVD risk, and the HDL level provided the greatest predictability in determining CVD events (28):
Comparatively, these results suggest that LDL-C or TG levels alone are lacking in predictability . . . indicating that parameters related to HDL-C are more effective in providing accurate risk assessment of CVD.
The superiority of using markers related to HDL was further emphasized in an analysis involving the LRC-CPPT population (29):
Men or women with similar ratios have similar risks for CHD regardless of their TC or LDL level. Men with similar changes in TC/HDL or LDL/HDL ratios have similar risks regardless of their change in TC or LDL level.
On the other hand, the ALLHAT trial could not find a relationship between baseline HDL and cardiovascular mortality. But baseline LDL was not associated with cardiovascular mortality either (30):
In univariable Cox regression models, age, black race, history of smoking, fasting triglycerides, and electrocardiographic left ventricular hypertrophy were associated with CV death, whereas baseline LDL, HDL, blood pressure, IFG, and BMI status were not.
In the CARDS trial, the association between LDL and coronary events appeared non-linear (similar event rates in the first two tertiles), and LDL did not have an association with strokes (31):
Neither the ApoB:ApoA-I nor any other lipoprotein concentration or ratio predicted the stroke outcome.
Lastly, LDL cholesterol was not a risk factor in the placebo group of JUPITER (32):
In the JUPITER trial population, recruited based on low LDL-c and elevated hsCRP, baseline LDL-c was not associated with incident CVD.
What About Secondary or Mixed Prevention Trials?
For the most part, we see the same.
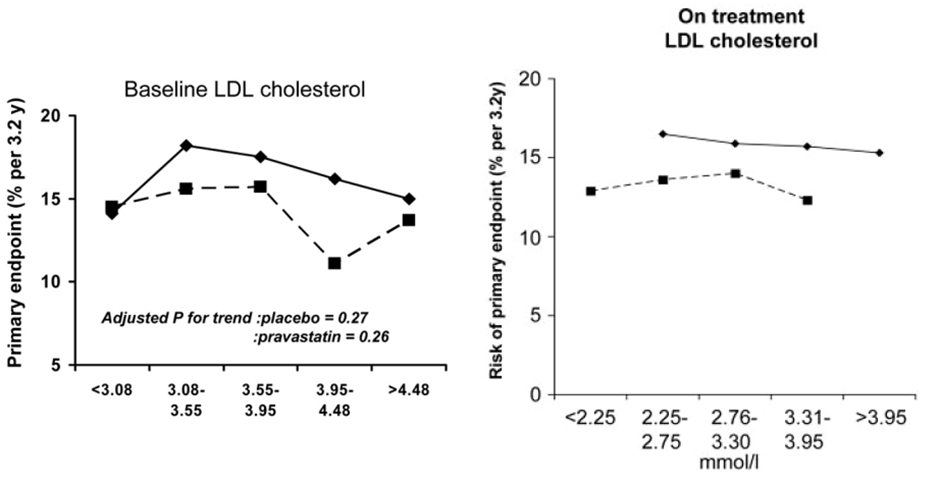
The CARE trial reported no significant association between the percentage or absolute change in LDL and coronary events. The researchers also found that the relationship between on-treatment LDL and coronary events is not a linear one (33):
A central finding of this study is that the relationship between LDL during treatment and coronary events is not a linear one but rather appears not to decline further below a concentration of ≈125 mg/dL. . . . we determined that the probability was high, 90%, that even a modest reduction in coronary events of 15% would have been detected.
Weak to no associations between LDL and cardiovascular outcomes were also found in the LIPID trial. The curves, if anything, suggested a non-linear relationship (34):

Nor was there any evidence of an association in the PROSPER trial (35):
HDLc appears to be a predictor not only of coronary risk but also of those who will benefit most from statin treatment. LDLc, in contrast, was not related to the risk of a coronary or cerebrovascular event, and neither a change in LDLc nor its achieved level on therapy was linked to risk reduction.
Likewise, in the SPARCL trial, baseline LDL was not related to major cardiovascular events (MCVEs). Only HDL, the LDL/HDL ratio, and the ApoB/ApoA1 ratio were (36):
Baseline LDL-C was not predictive of recurrent stroke or MCVEs regardless of stroke subtype or the presence of carotid stenosis . . . baseline HDL-C and LDL/HDL ratio, but not baseline LDL-C, were the strongest predictors of stroke and MCVEs.
Although these are secondary prevention statin trials, non-statin trials can produce similar results.
In the VA-HIT fibrate trial, only the increase in HDL was associated with a lower risk (37):
Neither triglyceride nor LDL-C levels at baseline or during the trial predicted CHD events.
 (Image: For the LDL graph on the left, there is no clear association. Note how flat the lines are compared to Silverman's secondary prevention meta-regression)
(Image: For the LDL graph on the left, there is no clear association. Note how flat the lines are compared to Silverman's secondary prevention meta-regression)
We could also cite many other findings.
For instance, based on combined data from three trials, Ridker and colleagues found no evidence of an association between LDL and cardiovascular events (38):
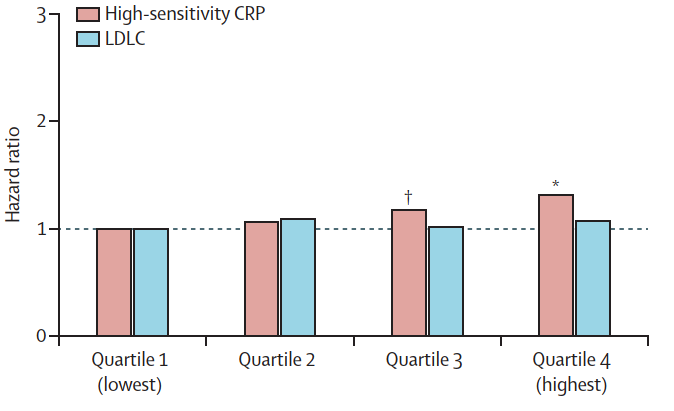
More importantly, when Nguyen and coworkers assessed the discriminatory accuracy of LDL in the SPRINT trial, they found that LDL was useless in predicting clinical outcomes (39):
The most important finding of this analysis is that no LDL-C threshold was associated more than another, with CV events. In the whole cohort, patients with LDL-C ≥ 160 mg/dl at the beginning of the study did not experience more CV events than those with LDL-C < 70 mg/dl.
Thus, Silverman's regression lines are not only inconsistent with a mass of data, but they also hide the fact that other risk markers — even other lipid markers (e.g., lipid ratios) — are consistently superior in predicting cardiovascular outcomes.
Acknowledging Bias
Three years after Silverman et al. published their paper, Toyota et al. (2019) conducted an analysis and found differing relationships between LDL and cardiovascular events depending on the lipid-lowering strategy (40).
Interestingly enough, they also stated:
The scientific validity of meta-regression analysis has not been firmly established.
But that wasn't the only time they expressed skepticism of meta-regressions.
Earlier this year, Toyota and colleagues (2023) again acknowledged the difficulties of meta-regressions, stating (41):
“The lower the better” hypothesis is basically derived from the meta-regression analyses of randomized controlled trials of “statins versus placebo”, “more versus less statins”, and “non-statin lipid-lowering therapy versus placebo”, suggesting that lower on-treatment LDL-C was associated with lower cardiovascular event rates. However, the results from the meta-regression analyses might not be robust, because there are big differences in the risk profiles of the patients enrolled in the individual trials, and in the intensity of lipid-lowering therapy between the trial arms and across the trials.
To address these biases (as much as they can), Toyota et al. conducted their own analysis of a secondary prevention trial. But the results did not support Silverman et al.
At the same statin dose (pitavastatin 1 mg/day) adjusting for a multitude of variables, there was no relationship between on-treatment LDL levels and cardiovascular outcomes:
The major findings of the present study were as follows: (1) very low on-treatment LDL-C level (<70 mg/dL) was not associated with lower cardiovascular event risk compared with moderately low on-treatment LDL-C level (70–100 mg/dL) in patients receiving the same doses of statins; (2) high LDL-C (≥100 mg/dL) was not associated with higher cardiovascular event risk compared with a moderately low LDL-C level (70–100 mg/dL) in the pitavastatin 1 mg/day stratum . . .
 (Image: Taken from reference 41. Note the lack of association in the 1 mg/day stratum. The higher risk for the grey bar in the 4 mg/day stratum was attributed to unreported non-adherence rather than LDL)
(Image: Taken from reference 41. Note the lack of association in the 1 mg/day stratum. The higher risk for the grey bar in the 4 mg/day stratum was attributed to unreported non-adherence rather than LDL)
Limitation #3: Lack of Robustness
As Toyota et al. noted above, results from meta-regressions "might not be robust."
For example, based on 25 statin trials, Silverman et al. claimed that each 1-mmol/L (38.7-mg/dL) reduction in the LDL-C level was associated with 23% relative risk reduction in cardiovascular events.
In 2013, however, Takagi and Umemoto also analyzed 25 statin trials (mostly the same trials) using flexible (not linear) meta-regression (42).
Contrary to Silverman's analysis, they found almost no relationship between the degree of LDL lowering and cardiovascular events beyond a 40 mg/dL decrease in the LDL-C level:
 (Image: The red curve fits the data better than a straight line)
(Image: The red curve fits the data better than a straight line)
Thus, just a few differences in trial and model selection could lead to inconsistent results.
As another example, take the following graph from Silverman's paper:

First, when Silverman et al. published their analysis, there were no PCSK9 trials designed to evaluate cardiovascular outcomes. Silverman et al. reported a 51% relative risk reduction for PCSK9 inhibitors based on post-hoc or "exploratory" outcomes. But in cardiovascular-outcome trials, the risk reduction is only around 15% (43).
Second, the ileal bypass trial in their graph is methodologically poor and confounded.
Therefore, if we use the correct estimate for the PCSK9 trials (with the correct weighted between-group difference in LDL), exclude the ileal bypass trial, and add the recent bempedoic acid trial instead (44), we get the following graph:
But this result is now more compatible with the Takagi and Umemoto model where the relative risk reduction appears weak to nonexistent beyond a certain LDL reduction.
Of note, we could apply a similar modification to Silverman's "nonstatin" analysis of eight trials. Since 2016, seven additional "nonstatin" trials seem to fit Silverman's inclusion criteria (CLEAR Outcomes, FOURIER, ODYSSEY OUTCOMES, SPIRE 1 and 2, HIJ-PROPER, and EWTOPIA 75).
If we add these trials to Silverman's "nonstatin" meta-regression, we get the following graph (P = 0.13):

In other words, by applying simple, reasonable changes to Silverman's analyses, there is no longer evidence that each 1-mmol/L reduction in LDL is associated with the same relative risk reduction (i.e., no evidence of a log-linear relationship) [10,45,46].
And this is not all.
Besides the above analyses, there are other published meta-regressions that are not consistent with Silverman's analysis.
For example, Werba et al. (2019) found that the relative risk reduction in major vascular events (per 1 mmol/L lower LDL) was different depending on the baseline LDL level (47). And Thomopoulos et al. (2015), Battaggia et al. (2018), Yang et al. (2022), and Byrne et al. (2022) could not find robust associations between LDL and cardiovascular outcomes (48-51).
 (Image: Taken from reference 51. No clear association between LDL and heart attacks on meta-regression)
(Image: Taken from reference 51. No clear association between LDL and heart attacks on meta-regression)
As Byrne and colleagues stated (51):
Because the meta-regression analysis yielded inconsistent results, we concluded that our meta-regression was inconclusive in proving or disproving an association between the magnitude of LDL-C reduction and the size of treatment effect.
Looking Forward to Part 2
The limitations of meta-regressions in this post are just part of the problem. In the next post, we will look at endpoint biases, biased inclusion/exclusion criteria, inadequate quality assessment, and conflicts of interest.
Go to Part 2.
References
1) Silverman, M. G., Ference, B. A., Im, K., Wiviott, S. D., Giugliano, R. P., Grundy, S. M., ... & Sabatine, M. S. (2016). Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. Jama, 316(12), 1289-1297.
2) Stegenga, J. (2022). Evidence of effectiveness. Studies in History and Philosophy of Science, 91, 288-295.
3) Ioannidis, J. P. (2010). Meta‐research: The art of getting it wrong. Research synthesis methods, 1(3‐4), 169-184.
4) Shrier, I., Boivin, J. F., Platt, R. W., Steele, R. J., Brophy, J. M., Carnevale, F., ... & Rossignol, M. (2008). The interpretation of systematic reviews with meta-analyses: an objective or subjective process?. BMC medical informatics and decision making, 8(1), 1-8.
5) Spineli, L. M., & Pandis, N. (2020). Problems and pitfalls in subgroup analysis and meta-regression. American journal of orthodontics and dentofacial orthopedics, 158(6), 901-904.
6) https://training.cochrane.org/handbook/current/chapter-10
7) Roumeliotis, S., Abd ElHafeez, S., Jager, K. J., Dekker, F. W., Stel, V. S., Pitino, A., ... & Tripepi, G. (2021). Be careful with ecological associations. Nephrology, 26(6), 501-505.
8) Cragg, J. J., Kramer, J. L., Borisoff, J. F., Patrick, D. M., & Ramer, M. S. (2019). Ecological fallacy as a novel risk factor for poor translation in neuroscience research: A systematic review and simulation study. European journal of clinical investigation, 49(2), e13045.
9) Bots, M. L., Taylor, A. J., Kastelein, J. J., Peters, S. A., Den Ruijter, H. M., Tegeler, C. H., ... & Grobbee, D. E. (2012). Rate of change in carotid intima–media thickness and vascular events: meta-analyses can not solve all the issues. A point of view.
10) Hayward, R. A., Hofer, T. P., & Vijan, S. (2006). Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Annals of internal medicine, 145(7), 520-530.
11) Riley, R. D., Tierney, J. F., & Stewart, L. A. (Eds.). (2021). Individual participant data meta-analysis: a handbook for healthcare research. John Wiley & Sons.
12) Rao, G., Lopez-Jimenez, F., Boyd, J., D’Amico, F., Durant, N. H., Hlatky, M. A., ... & Wessel, J. (2017). Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation, 136(10), e172-e194.
13) Baker, W. L., Michael White, C., Cappelleri, J. C., Kluger, J., Coleman, C. I., & From the Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. (2009). Understanding heterogeneity in meta‐analysis: the role of meta‐regression. International journal of clinical practice, 63(10), 1426-1434.
14) Charland, S. L., & Stanek, E. J. (2014). Sigmoidal Maximal Effect Modeling of Low‐Density Lipoprotein Cholesterol Concentration and Annual Incidence of Coronary Heart Disease Events in Secondary Prevention Trials. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 34(5), 452-463.
15) Sturmberg, J. P. (2020). From probability to believability. Journal of Evaluation in Clinical Practice, 26(4), 1081-1086.
16) Okuyama, H., Hamazaki, T., Hama, R., Ogushi, Y., Kobayashi, T., Ohara, N., & Uchino, H. (2018). A critical review of the consensus statement from the European Atherosclerosis Society Consensus Panel 2017. Pharmacology, 101(3-4), 184-218.
17) Yuan, S., Huang, X., Ma, W., Yang, R., Xu, F., Han, D., ... & Lyu, J. (2023). Associations of HDL-C/LDL-C with myocardial infarction, all-cause mortality, haemorrhagic stroke and ischaemic stroke: a longitudinal study based on 384 093 participants from the UK Biobank. Stroke and Vascular Neurology, 8(2).
18) Liu, Y., Liu, F., Zhang, L., Li, J., Kang, W., Cao, M., ... & Song, F. (2021). Association between low density lipoprotein cholesterol and all-cause mortality: results from the NHANES 1999–2014. Scientific Reports, 11(1), 1-12.
19) Penson, P. E., Long, D. L., Howard, G., Toth, P. P., Muntner, P., Howard, V. J., ... & Banach, M. (2018). Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C-reactive protein, and health outcomes in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study. European Heart Journal, 39(40), 3641-3653.
20) Mortensen, M. B., Dzaye, O., Bøtker, H. E., Jensen, J. M., Maeng, M., Bentzon, J. F., ... & Nørgaard, B. L. (2023). Low-Density Lipoprotein Cholesterol Is Predominantly Associated With Atherosclerotic Cardiovascular Disease Events in Patients With Evidence of Coronary Atherosclerosis: The Western Denmark Heart Registry. Circulation, 147(14), 1053-1063.
21) Yi, S. W., An, S. J., Park, H. B., Yi, J. J., & Ohrr, H. (2022). Association between low-density lipoprotein cholesterol and cardiovascular mortality in statin non-users: a prospective cohort study in 14.9 million Korean adults. International Journal of Epidemiology.
22) Nanna, M. G., Navar, A. M., Wojdyla, D., & Peterson, E. D. (2019). The association between low‐density lipoprotein cholesterol and incident atherosclerotic cardiovascular disease in older adults: results from the national institutes of health pooled cohorts. Journal of the American Geriatrics Society, 67(12), 2560-2567.
23) Eliasson, B., Gudbjörnsdottir, S., Zethelius, B., Eeg-Olofsson, K., & Cederholm, J. (2014). LDL-cholesterol versus non-HDL-to-HDL-cholesterol ratio and risk for coronary heart disease in type 2 diabetes. European journal of preventive cardiology, 21(11), 1420-1428.
24) Shepherd, J., & Park, J. S. (1998). Prevention of heart disease: is LDL reduction the outcome of choice? No, there is more. Value in Health, 1(2), 120-124.
25) Ford, I., Shah, A. S., Zhang, R., McAllister, D. A., Strachan, F. E., Caslake, M., ... & Mills, N. L. (2016). High-sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. Journal of the American College of Cardiology, 68(25), 2719-2728.
26) Gotto Jr, A. M., Whitney, E., Stein, E. A., Shapiro, D. R., Clearfield, M., Weis, S., ... & De Cani, J. S. (2000). Relation between baseline and on-treatment lipid parameters and first acute major coronary events in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Circulation, 101(5), 477-484.
27) Sakamoto, T., & Ogawa, H. (2010). " Just Make It Lower" Is an Alternative Strategy of Lipid-Lowering Therapy With Statins in Japanese Patients–LDL-Cholesterol: The Lower, the Better; Is It True for Asians?(Con)–. Circulation journal, 74(8), 1731-1741.
28) Mizuno, K., Nakaya, N., Teramoto, T., Yokoyama, S., Ohashi, Y., Ueki, A., ... & Nakamura, H. (2012). Usefulness of LDL-C-related parameters to predict cardiovascular risk and effect of pravastatin in mild-to-moderate hypercholesterolemia. Journal of Atherosclerosis and Thrombosis, 19(2), 176-185.
29) Natarajan, S., Glick, H., Criqui, M., Horowitz, D., Lipsitz, S. R., & Kinosian, B. (2003). Cholesterol measures to identify and treat individuals at risk for coronary heart disease. American journal of preventive medicine, 25(1), 50-57.
30) Shah, R. V., Abbasi, S. A., Yamal, J. M., Davis, B. R., Barzilay, J., Einhorn, P. T., ... & ALLHAT Collaborative Research Group. (2014). Impaired fasting glucose and body mass index as determinants of mortality in ALLHAT: is the obesity paradox real?. The Journal of Clinical Hypertension, 16(6), 451-458.
31) Charlton-Menys, V., Betteridge, D. J., Colhoun, H., Fuller, J., France, M., Hitman, G. A., ... & Durrington, P. N. (2009). Apolipoproteins, cardiovascular risk and statin response in type 2 diabetes: the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia, 52, 218-225.
32) Mora, S., Caulfield, M. P., Wohlgemuth, J., Chen, Z., Superko, H. R., Rowland, C. M., ... & Krauss, R. M. (2015). Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: the justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) trial. Circulation, 132(23), 2220-2229.
33) Sacks, F. M., Moye, L. A., Davis, B. R., Cole, T. G., Rouleau, J. L., Nash, D. T., ... & Braunwald, E. (1998). Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation, 97(15), 1446-1452.477-484.
34) Hilvo, M., Meikle, P. J., Pedersen, E. R., Tell, G. S., Dhar, I., Brenner, H., ... & Laaksonen, R. (2020). Development and validation of a ceramide-and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. European heart journal, 41(3), 371-380.
35) Packard, C. J., Ford, I., Robertson, M., Shepherd, J., Blauw, G. J., Murphy, M. B., ... & Westendorp, R. G. (2005). Plasma lipoproteins and apolipoproteins as predictors of cardiovascular risk and treatment benefit in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Circulation, 112(20), 3058-3065.
36) Amarenco, P., Goldstein, L. B., Callahan III, A., Sillesen, H., Hennerici, M. G., O’Neill, B. J., ... & SPARCL Investigators. (2009). Baseline blood pressure, low-and high-density lipoproteins, and triglycerides and the risk of vascular events in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Atherosclerosis, 204(2), 515-520.
37) Robins, S. J., Collins, D., Wittes, J. T., Papademetriou, V., Deedwania, P. C., Schaefer, E. J., ... & VA-HIT Study Group. (2001). Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. Jama, 285(12), 1585-1591.
38) Ridker, P. M., Bhatt, D. L., Pradhan, A. D., Glynn, R. J., MacFadyen, J. G., & Nissen, S. E. (2023). Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. The Lancet, 401(10384), 1293-1301.
39) Nguyen, L. S., Procopi, N., Salem, J. E., Squara, P., & Funck-Brentano, C. (2019). Relation between baseline LDL-cholesterol and cardiovascular outcomes in high cardiovascular risk hypertensive patients: A post-hoc SPRINT data analysis. International Journal of Cardiology, 286, 159-161.
40) Toyota, T., Morimoto, T., Yamashita, Y., Shiomi, H., Kato, T., Makiyama, T., ... & Kimura, T. (2019). More-versus less-intensive lipid-lowering therapy: systematic review and meta-analysis. Circulation: Cardiovascular Quality and Outcomes, 12(8), e005460.
41) Toyota, T., Morimoto, T., Iimuro, S., Fujita, R., Iwata, H., Miyauchi, K., ... & Kimura, T. (2023). Low-Density Lipoprotein Cholesterol Levels on Statins and Cardiovascular Event Risk in Stable Coronary Artery Disease―An Observation From the REAL-CAD Study―. Circulation Journal, 87(2), 360-367.
42) Takagi, H., Umemoto, T., & ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. (2013). Limit to benefits of large reductions in low-density lipoprotein cholesterol levels: use of fractional polynomials to assess the effect of low-density lipoprotein cholesterol level reduction in metaregression of large statin randomized trials. JAMA internal medicine, 173(11), 1028-1029.
43) Dicembrini, I., Giannini, S., Ragghianti, B., Mannucci, E., & Monami, M. (2019). Effects of PCSK9 inhibitors on LDL cholesterol, cardiovascular morbidity and all-cause mortality: a systematic review and meta-analysis of randomized controlled trials. Journal of endocrinological investigation, 42, 1029-1039.
44) Nissen, S. E., Lincoff, A. M., Brennan, D., Ray, K. K., Mason, D., Kastelein, J. J., ... & Nicholls, S. J. (2023). Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. New England Journal of Medicine, 388(15), 1353-1364.
45) https://www.ti.ubc.ca/2021/06/13/130-evidence-for-statins-in-people-over-70/
46) Lindblad, A. J., Kolber, M. R., Garrison, S., Cotton, C., & Allan, G. M. (2015). Prevention and Management of Cardiovascular Disease Risk in Primary Care: Evidence Review of 12 Key Clinical Questions. Alberta College of Family Physicians.
47) Werba, J. P., Vigo, L. M., Veglia, F., Marenzi, G., Tremoli, E., & Baldassarre, D. (2019). Trials in “True” Dyslipidemic Patients Are Urged to Reconsider Comprehensive Lipid Management as a Means to Reduce Residual Cardiovascular Risk. Clinical Pharmacology & Therapeutics, 106(5), 960-967.
48) Thomopoulos, C., Skalis, G., Michalopoulou, H., Tsioufis, C., & Makris, T. (2015). Effect of low‐density lipoprotein cholesterol lowering by ezetimibe/simvastatin on outcome incidence: Overview, meta‐analyses, and meta‐regression analyses of randomized trials. Clinical Cardiology, 38(12), 763-769.
49) Battaggia, A., Scalisi, A., & Donzelli, A. (2018). The systematic review of randomized controlled trials of PCSK9 antibodies challenges their “efficacy breakthrough” and the “lower, the better” theory. Current Medical Research and Opinion, 34(10), 1725-1730.
50) Yang, W., Cai, X., Lin, C., Lv, F., Zhu, X., Han, X., & Ji, L. (2022). Reduction of C-reactive protein, low-density lipoprotein cholesterol, and its relationship with cardiovascular events of different lipid-lowering therapies: A systematic review and meta-analysis of randomized controlled trials. Medicine, 101(37), e30563.
51) Byrne, P., Demasi, M., Jones, M., Smith, S. M., O’Brien, K. K., & DuBroff, R. (2022). Evaluating the association between low-density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta-analysis. JAMA internal medicine.
